Topics to be learn :
- Elements and their classification
- Dobereiner’s Triads
- Newlands Law of Octaves
- Mendeleev's Periodic Table
- Modern Periodic Table
Types of Matter
- Solid: Has a fixed shape and volume.
- Liquid: Takes the shape of its container but has a fixed volume.
- Gas: Fills the entire space of its container and has neither a fixed shape nor volume.
- Plasma: A state of matter where the gas phase is energized until atomic electrons are no longer associated with any particular atomic nucleus.
Types of Elements
- Metals: Elements that are typically shiny, malleable, and good conductors of electricity and heat.
- Nonmetals: Elements that are generally not shiny, brittle, and poor conductors of electricity and heat.
- Metalloids: Elements with properties intermediate between metals and nonmetals.
Atoms
- Atoms: The smallest particles of matter that retain the properties of an element.
Difference Between Molecules of Elements and Compounds
Dobereiner’s Triads
- Johann Wolfgang Döbereiner (1817, German scientist) showed that the properties of elements are related to their atomic masses.
- He created triads, groups of three elements with similar chemical properties.
- The elements in a triad were arranged in increasing order of atomic mass.
- The atomic mass of the middle element was approximately equal to the mean of the atomic masses of the other two elements.
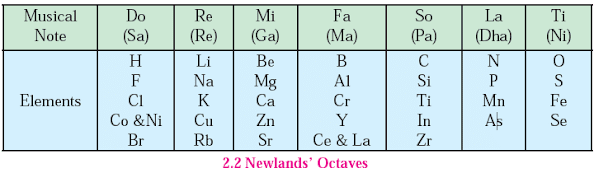
Newlands’ Law of Octaves
- Year: 1866
- Concept: Newlands arranged elements in increasing order of their atomic masses and observed that every eighth element had properties similar to the first.
- Example: Sodium is the eighth element from lithium, and both share similar properties.
Limitations of Newlands’ Octaves
- Limited to Calcium: Newlands could arrange elements only up to calcium (20 elements out of the total 56 known at that time).
- Inconsistent Pattern: After calcium, every eighth element did not possess properties similar to the first.
- Discovery of New Elements: Only 56 elements were known during Newlands’ time, but many more were discovered later.
- Inaccurate Placement: Newlands placed two elements in the same position even if they had different properties.
- Example: Iron was placed far away from cobalt and nickel, despite their similar properties.
- Absence of Inert Gases: The periodic table did not include inert gases as they were not discovered at that time.
Mendeleev’s Periodic Table
Key Idea: Mendeleev used atomic mass as the fundamental property to classify elements.
- Arrangement: He arranged 63 known elements in increasing order of atomic masses.
- Periodic Table Transformation: Elements were organized according to their physical and chemical properties.
- Repetition of Properties: Mendeleev observed that elements with similar properties repeat after a definite interval.
Mendeleev’s Periodic Law
- Law: The elements’ physical and chemical properties are a periodic function of their atomic masses.
Structure of the Periodic Table
- Groups: The vertical columns in the periodic table.
- Periods: The horizontal rows in the periodic table.
a) Revision of Atomic Masses: Mendeleev revised the atomic masses of some elements to fit them better in the periodic table based on their properties.
- Example: The atomic mass of beryllium was corrected from 14.09 to 9.4, placing it correctly before boron.
b) Prediction of Unknown Elements: Mendeleev left vacant spaces in the periodic table for elements yet to be discovered.
- He predicted the existence and properties of elements he called eka-boron, eka-aluminium, and eka-silicon.
- These elements were later discovered as scandium (Sc), gallium (Ga), and germanium (Ge), and their properties matched Mendeleev’s predictions.
c) Recognition of Mendeleev’s Periodic Table: The accurate predictions of undiscovered elements convinced the scientific community of the table’s importance.
d) Inclusion of Noble Gases: When noble gases like helium, neon, and argon were discovered, Mendeleev created a ‘zero group’ in the periodic table to accommodate them without disturbing the existing structure.
Demerits of Mendeleev’s Periodic Table
a) Ambiguity in Element Positioning: Elements like cobalt (Co) and nickel (Ni) have the same whole number atomic mass, leading to uncertainty in their sequence.
b) Challenge of Isotopes: Isotopes were discovered after Mendeleev’s table, posing a challenge as they have the same chemical properties but different atomic masses, making their placement difficult.
c) Non-Uniform Rise in Atomic Mass: The rise in atomic mass was not uniform, making it impossible to predict how many elements could be discovered between two heavy elements.
d) Position of Hydrogen: Hydrogen shows similarities with both halogens (Group VII) and alkali metals (Group I).
- Chemical Properties: Molecular formula of hydrogen: H₂, Molecular formulas of halogens: F₂, Cl₂
- Similarity with Alkali Metals: Hydrogen forms compounds with chlorine and oxygen similar to alkali metals (e.g., NaCl, HCl).
- Dilemma: It is difficult to decide the correct position for hydrogen in the periodic table—whether with alkali metals (Group I) or halogens (Group VII).
- Discovery of Electron: Led to exploring the relationship between an atom's electron number and its atomic number.
- Atomic Number in Mendeleev’s Table: Originally indicated only the serial number of the element.
- Henry Moseley’s Contribution: Showed that atomic number is the most fundamental property of an element, not atomic mass.
- Modern Periodic Statement: The chemical and physical properties of elements are a periodic function of their atomic numbers.
Structure of the Modern Periodic Table
- Arrangement: Elements are arranged in increasing order of their atomic numbers.
- Periods: Seven horizontal rows numbered 1 to 7.
- Groups: Eighteen vertical columns numbered 1 to 18.
- Boxes: The arrangement of periods and groups forms boxes, each representing one element, with the atomic number displayed at the top.
- Lanthanide and Actinide Series: Two series of elements placed separately at the bottom, representing the lanthanide and actinide series.
- Total Elements: There are 118 boxes in the periodic table, including those for the lanthanides and actinides.
Blocks in the Modern Periodic TableFour Blocks: Elements are divided into four blocks: s-block, p-block, d-block, and f-block.
- Groups: 1 and 2, plus hydrogen.
- Outer Shell Electrons: 1 or 2 electrons.
- Properties: Except for hydrogen, all s-block elements are metals.
- Groups: 13 to 18.
- Outer Shell Electrons: 3 to 8 electrons.
- Properties: Includes a few metals, all metalloids, and all nonmetals.
- Groups: 3 to 12.
- Also Known As: Transition elements.
- Series: Lanthanides and actinides at the bottom.
- Metalloids: Lie along the border of the zig-zag line.
- Metals: Positioned on the left side of the zig-zag line.
- Nonmetals: Positioned on the right side of the zig-zag line.
- Determines the placement of elements in specific groups and periods in the periodic table.
- Neighboring elements in a period have slightly different properties, while distant elements differ widely in their properties.
- Elements within the same group show similarity and gradation in their properties.
Groups and Electronic Configuration
- Group 1 (Alkali Metals): All elements have the same number of valence electrons.
- Group 2 (Alkaline Earth Metals): Elements like beryllium (Be), magnesium (Mg), and calcium (Ca) have 2 valence electrons.
- Group 17 (Halogens): Elements like fluorine (F) and chlorine (Cl) have 7 valence electrons.
- Moving top to bottom within a group adds one electronic shell each time.
- The electronic configuration of the outermost shell is characteristic of a particular group.
Structure of the Modern Periodic Table
- Elements are arranged in increasing order of their atomic numbers.
- Vertical Columns (Groups): There are 18 groups. Elements in the same group exhibit similar chemical properties and a gradual change in these properties.
- Horizontal Rows (Periods): There are 7 periods. Properties of elements change gradually from one end to the other within a period.
Horizontal Rows: There are seven periods in the modern periodic table.
Valency Variation:
- The change in valency of elements across a period is based on their electronic configuration.
- In a period, the number of valence electrons increases by one as you move from left to right, while the number of electronic shells remains constant.
- Example:
- Second Period: Elements like Li, Be, B, C, N, O, F, and Ne have electrons in two shells (K and L).
- Third Period: Elements like Na, Mg, Al, Si, P, S, Cl, and Ar have electrons in three shells (K, L, and M).
Chemical Reactivity: Determined by the number of valence electrons and the shell number of the valence shell.
Periodic Trends
- Definition: Regular patterns observed in the properties of elements across a period or group in the periodic table are called periodic trends.
- Key Properties Studied: Valency, Atomic Size, Metallic-Nonmetallic Character
Distinguish Between Groups and Periods
Valency
- Definition: The valency of an element is determined by the number of valence electrons present in the outermost shell of its atoms.
Electron Capacity of Shells
- Formula: The maximum number of electrons that can be accommodated in a shell is given by 2n², where n is the shell number.
- Maximum Electron Capacity: 32 electrons.
Atomic Size
- Definition: The size of an atom is indicated by its atomic radius, which is the distance between the nucleus of the atom and its outermost shell.
- Unit: Atomic radius is expressed in picometres (pm), where 1 pm = 10⁻¹² meters.
- More shells = Larger atomic size.
- Down a Group: Atomic size increases as a new shell is added, increasing the distance between the outermost electrons and the nucleus.
- Across a Period: Atomic radius decreases from left to right. This is due to the increase in nuclear charge, which pulls the electrons closer to the nucleus, decreasing the atomic size.
Key Points:
- Down a Group: Atomic size increases.
- Across a Period: Atomic size decreases.
Metallic-Nonmetallic Character
a) Metallic Elements:- Found on the left side of the periodic table (e.g., sodium, magnesium).
- Tend to lose valence electrons and form cations (positive ions). This property is known as electropositivity.
- Found on the right side of the periodic table (e.g., sulfur, chlorine).
- Tend to gain electrons and form anions (negative ions). This property is known as electronegativity.
- Located along the zig-zag line that separates metals from nonmetals (e.g., silicon).
- Have properties intermediate between metals and nonmetals.
Trends:
- Down a Group:
- Electropositivity increases.
- Electronegativity decreases.
- Across a Period:
- Electronegativity increases.
- Electropositivity decreases.
Reactivity: The greater the electropositivity or electronegativity of an element, the higher its reactivity.
Periodic Trend in Metallic Character
- Definition: The metallic character of an element refers to its tendency to lose electrons and form cations. This character is influenced by the element's position in the periodic table.
Down a Group:
- A new shell is added as you move down, increasing the distance between the nucleus and the valence electrons.
- Effective Nuclear Charge: Decreases due to the increased distance, leading to a weaker attraction between the nucleus and the valence electrons.
- Result: The atom's tendency to lose electrons increases, enhancing its metallic character.
- Stability: The penultimate shell, being a complete octet, provides special stability to the resulting cation.
Trend in a Period:
Left to Right Across a Period:- The outermost shell remains the same, but the positive charge on the nucleus increases.
- Effective Nuclear Charge: Increases, pulling valence electrons closer and making them harder to lose.
- Electronegativity: Increases, leading to a decrease in metallic character and an increase in nonmetallic character.
Summary:
- Down a Group: Metallic character increases.
- Across a Period (Left to Right): Metallic character decreases, and nonmetallic character increases.
Gradation in the Halogen Family (Group 17)
Halogens: The halogen family includes fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). All halogens have the general molecular formula X₂.
Physical State: Fluorine (F₂) and Chlorine (Cl₂): Gases, Bromine (Br₂): Liquid, Iodine (I₂): Solid.
Trend: A gradation in physical states is observed as you move down the group, transitioning from gases to liquid, and finally to a solid.
Reaction of Alkaline Earth Metals with Water
General Reaction:
Trend in Group 2 (Alkaline Earth Metals):
- Elements: Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba).
- Reactivity: Increases as you move down the group.
- Beryllium (Be): Does not react with water.
- Magnesium (Mg): Reacts with steam.
- Calcium (Ca), Strontium (Sr), Barium (Ba): React with water at room temperature with increasing rates as you move down the group.












0 Comments